Development of pharmacokinetic analysis method for virus therapeutics using mass spectrometry
Abstract
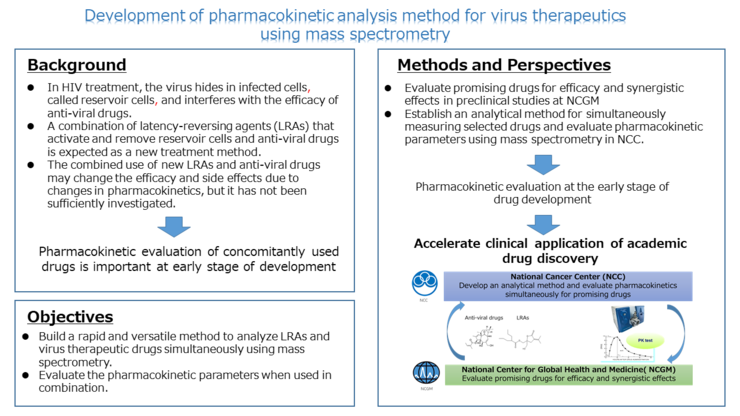
In the treatment of HIV, antiviral drugs are effective in suppressing viral growth. However, viruses can hide in infected cells, called HIV reservoir cells, and hinder drug efficacy. In recent years, concomitant use of anti-viral drugs and latency-reversing agents (LRAs), which can activate and eliminate HIV reservoir cells, is undergoing research. Furthermore, synergistic effects can also be expected by combining LRAs of different mechanisms. Most studies are focusing on drug efficacy and side-effects, however, pharmacokinetic analysis regarding the combined use of these drugs has not been fully investigated.
The purpose of this study is to establish a simple and rapid method for pharmacokinetic analysis of both anti-viral drugs and LRAs in plasma and tissues. The activity of multiple candidate LRAs and anti-HIV drugs will be confirmed both in vitro and in vivo at the National Center for Global Health and Medicine (NCGM). The National Cancer Center (NCC) will build an analytical method to measure drug concentration using mass spectrometry. After validation of the analytical method, we will perform the co-administration of anti-viral drugs and candidate LRAs to experimental animals, and evaluate pharmacokinetics in plasma and tissues.
This research integrates the pharmacokinetic platform and technology in NCC and the development platform of viral therapeutics at NCGM. It is expected to accelerate academic drug discovery research, and deliver new, promising drugs to patients more quickly.
Perspectives
- Promising agents that have synergistic effects will be selected from multiple candidate compounds to elicit maximum clinical effect.
- By collaboration with NCGM and NCC, we will involve both efficacy and pharmacokinetic evaluation at the early stage of research, consequently accelerating the academic discovery of new therapeutic agents and clinical application for viral diseases.
Comments from principal researcher
The pharmacokinetics of a drug is closely related to its efficacy and side-effects in vivo. There are often cases of inadequate efficacy or unexpected side-effects due to a poor pharmacokinetic evaluation for a drug. There are many factors, including enzymes and concomitant drugs, that might affect the pharmacokinetics. In this research, we will build an analytical method to measure and evaluate the pharmacokinetics of viral therapeutic agents that are expected to be used in combination at an early stage of development. We hope to promote the development of new promising drugs from academia for clinical application.
Shoraku Ryu, Project Researcher, Division of Molecular Pharmacology, Department of Pharmacology and Therapeutics, National Cancer Center Research Institute.