Basic Policies & Organization
Basic Policies of Japan Health Research Promotion Bureau
Mission of Japan Health Research Promotion Bureau
Japan Health Research Promotion Bureau (JH) was launched in April 2020, aiming to create innovation with an eye toward world-class R&D and medical care by collecting and coordinating resources and information of six national centers for advanced and specialized medicine in Japan (NCs)* and by promoting organic and functional cooperation and collaboration between the six NCs taking full advantage of specialization of individual research institutes. It also aims to contribute to enhancing Japan's clinical research capabilities.
*The six NCs are the National Cancer Center Japan, the National Cerebral and Cardiovascular Center, the National Center of Neurology and Psychiatry, the National Center for Global Health and Medicine, the National Center for Child Health and Development, and the National Center for Geriatrics and Gerontology.
Basic Policy
To achieve the above-mentioned mission, we at JH will:
- Support and strengthen the functions of R&D in response to new needs;
- Support and strengthen R&D efforts in the fields in which the effective promotion of R&D is expected through close cooperation between the six NCs; and
- Support and strengthen the application of research achievements to clinical practice in the six NCs.
Management System
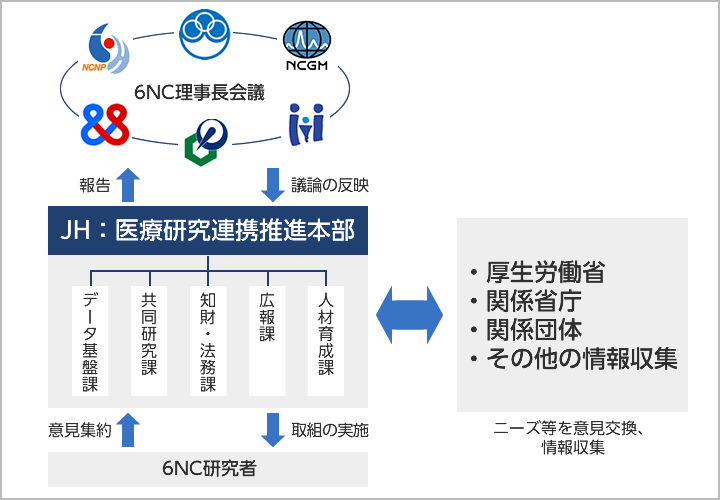
Specific projects to be implemented in the future
Creating and strengthening the infrastructure for data collection and coordination
- Constructing an information infrastructure to support the development of a registry by acquiring disease information via electronic medical charts
- Creating rules on the use of registry information
- Promoting coordination with the disease registries of other research institutes
Promoting collaborative research
- Promoting social medicine
- Promoting cross-disease clinical research (sharing of biostatistics)
- Promoting research utilizing biobanks and disease registries
- Constructing collaborative research facilities with expensive equipment (for NGS, omics analysis, imaging, PK/PD) (including joint employment of shared)
- Making joint bioinformaticians research proposals to AMED (Japan Agency for Medical Research and Development) and other institutes
- Promoting and participating in collaborative research with private enterprises
- Promoting the efficiency of research (including joint purchase of reagent kits and equipment, the development of common manuals regarding infection prevention, and sharing of online journal subscription)
Public relations
- Creating a web page
- Disseminating research results and utilizing social networking service
- Holding media seminars and public lectures
- Strengthening the function for policy recommendation
Intellectual property & legal affairs
- Reinforcing the acquisition and management of intellectual property rights
- Strengthening support for conclusion of collaborative research contracts
- Providing support and advice on clinical research from a legal perspective
- Promoting the establishment of collaborative labs with private enterprises
Research training and career
- Supporting human resource development who can play a leading role in promoting collaborative research for cross-disease analysis
- Supporting the fostering of data scientists (bioinformaticians, biostatisticians, etc.)
- Supporting the fostering of human resources in social medicine who can play an active role in public health, epidemiology, and policy recommendation
- Providing support to young physicians studying for a degree or professional qualifications (cooperation system of graduate schools, etc.)
